Lately, I’ve had great apes on my mind. Psychiatrist Martin Brüne’s work treating psychopathy in retired laboratory chimps – the topic of my Q&A article – got me thinking about some even slipperier issues. Brüne opened his talk at the annual American Association for the Advancement of Science meeting in February with what he called a “Freudian statement:” Our growing knowledge of the similarities between apes and ourselves comes as “a blow to human self-centeredness,” he said. Our closest living relative, it turns out, has many of the qualities we once considered uniquely human. Chimps use tools, recognize themselves in mirrors and even wage war. “Do we need to add another blow by claiming not even mental illness and suffering is reserved for our own species?” Brüne asked.
It’s a big question. And while Dr. Brune’s time at the AAP chimpanzee sanctuary in the Netherlands might make him more qualified than the average bipedal primate to speculate about our evolutionary uniqueness (or lack thereof), observational study isn’t enough. The scientific study of human uniqueness must go to the source: DNA.
This month I got a fascinating glimpse at the work of computational biologist Ed Green on April 3 through the new Santa Cruz lecture series Science on Tap [https://sites.google.com/site/ucscwise/science-on-tap]. (Santa Cruzers: Science! Crepes! Beer! Monthly!) Green hopes his work in paleogenomics might help explain when and how we became the curious animals we are today.
Scientists can now sequence a whole genome in a matter of days and make detailed comparisons between individuals. But comparing humans and chimps this way isn’t very meaningful. There are about 35 million differences between our two genomes. “Most of it is just noise,” Green told the audience. “So it’s a hard problem, to look in this noise and find the real differences. It would be easier if there were something alive that was more closely related to us than chimpanzees.”
There were, of course, such creatures: our extinct relatives. Green and his colleagues analyze ancient DNA from the bones of Neanderthals. Much of his work to date has examined how the human lineage dispersed across continents – in Green’s words, “where humans were, and who were they having sex with at different point in the past.” But recently, he has focused on describing how we have developed biologically since our lineage split from Neanderthals.
As we reproduce, random recombination mixes up the DNA of our ancestors. We pass their genes along as fragments of our offspring’s chromosomes in a process Green declared to be “really freaking complicated.” If you look back 200 years, you have about 1000 ancestors, and only one of them gave you the particular segment of DNA at any given point on your chromosome.
While any two humans on earth will share some common ancestor about 400,000 years ago, we all have a common ancestor population with Neanderthals more recently – 300,000 years ago, Green said. That ancestor wasn’t human, but its genetic variation filtered through the centuries to influence both the Neanderthal genome and our own.
Green is interested in points on our genome were all humans had a common ancestor more recently than any human had a common ancestor with Neanderthals. (You may need to chew on that syntax for a moment, as I did.) The idea is that these are the places that make us unique from Neanderthals; these more recent developments distinguish us from our non-human relatives.
“These are very interesting, very important places in our genome that makes us uniquely human, even with respect to the Neanderthals” Green said.

Painting from Chauvet Cave in France, believed to be about 30,000 years old, suggest the roots of human artistry. (Photo: HTO/Wikimedia commons)
These DNA regions arose by useful mutations that increased the amount of offspring an individual could have. Sometimes, a mutation would be so advantageous that anyone without it didn’t survive – a phenomenon called a hard sweep. “It wipes clean the slate of genetic variation in that region of the genome for some time,” Green says. Then when new variation develops from this point forward, there will be a region of the genome where our variation isn’t shared with Neanderthals.
Green suggested that our capacity for art – a skill early humans demonstrated on the walls of caves about 30,000 years ago – could be one such unique trait hidden somewhere in our genes. He hasn’t identified the mutation that made us artistic, and admits that genetics still has only the crudest understanding of how traits map to genes. (Who could say whether the artsy types have some reproductive advantage anyway?) But he did describe one case study that demonstrates his idea.
A certain region of our DNA holds a gene called RunX2, and people missing one copy of this gene have a condition called cleidocranial dysplasia [http://ghr.nlm.nih.gov/condition/cleidocranial-dysplasia]. Some of the symptoms, like a bell-shaped rib cage and a protrusion of the face called frontal bossing, are reminiscent of a Neanderthal body type. According to Green, this condition suggests that the RunX2 gene developed in important ways as we evolved away from our Neanderthal relatives.
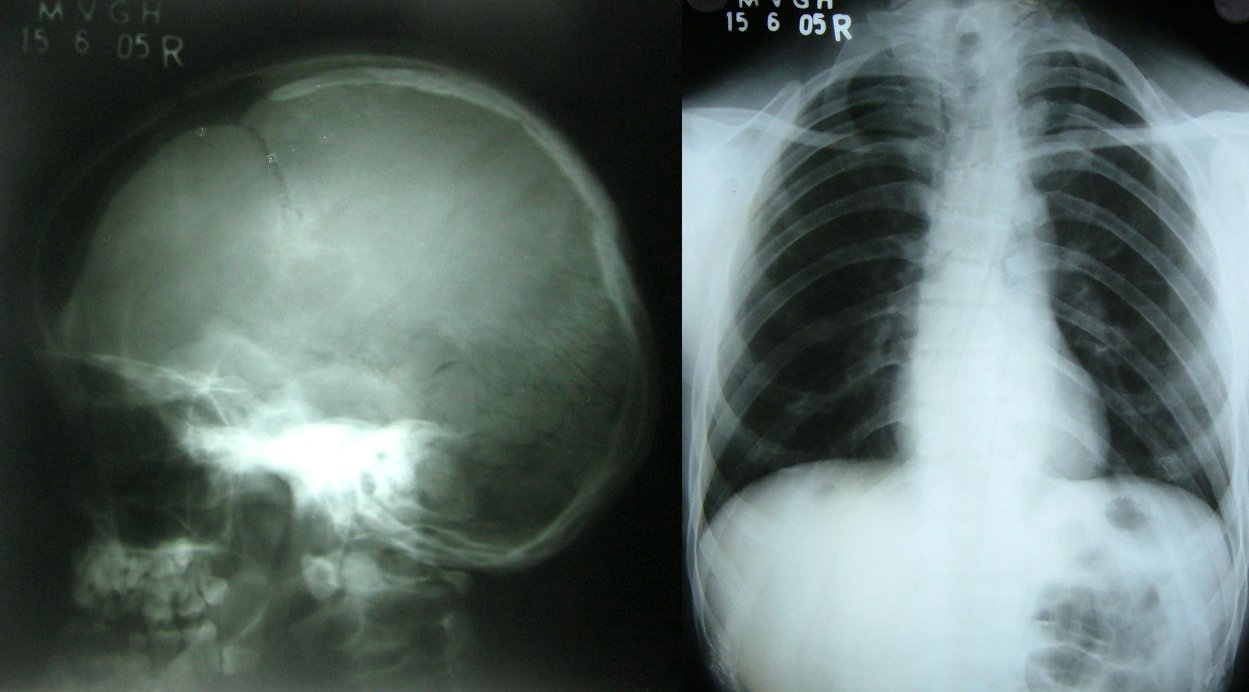
Bossing of the skull and bell-shaped ribcage in cleidocranial dysplasia (photos: Garg RK, Agrawal P, Wikimedia common
The regions of the genome that code for the complexities of our mental life – and the ones that might make us vulnerable to mental illness – are still largely mysterious to Green and his colleagues. But if other traits work like RunX2, our mental split from what came before us could be hidden in the genome somewhere, too.
So there’s one little piece of the story: we have a way to (possibly) track (aspects of) our specialness through time by comparing ourselves (in a specific, limited way) to relatives long vanished. For me, genomics sparks a big appetite for meaning, then tosses out only meager crumbs of fact. But my little hominid brain still finds comfort in the idea that we are, with baby steps, getting somewhere.







Comments are closed.